The Lethal Hazards of OxygenOxygen is a dangerous thing, a deadly poison which ages our bodies, prom
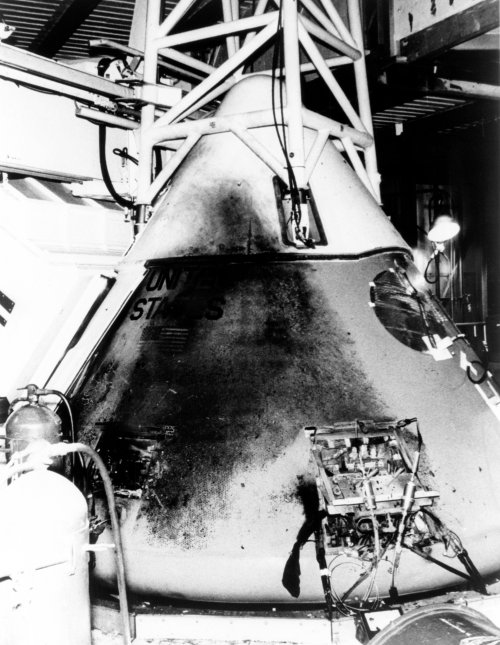
The Lethal Hazards of OxygenOxygen is a dangerous thing, a deadly poison which ages our bodies, promotes the growth of cancerous free radicals, and can even rust or tarnish metal. Alas, we humans are oxygen junkies, jonesin’ for at least 21% FIO2 every moment of our lives. With every breath we take, our addiction to oxygen is slowly destroying us, hastening the moment of our final demise. At high concentration oxygen can be especially dangerous. More oxygen means less nitrogen, a precious gas which is needed to keep the alveoli in our lungs inflated. If oxygen pushes out healthy nitrogen levels, a situation called absorption atelectasis occurs where the alveoli collapse from a lack of nitrogen, thus leading to decreased gas exchange. At levels 100% FIO2 oxygen can poison the body over time, causing retinal damage, inflammation of the lungs and airways, and toxicity of the central nervous system resulting in seizures. Thus, History Teacher/Respiratory Therapist peashooter knows that critically ill patients on high levels of oxygen must be titrated down to lower levels as soon as medically feasible. One other curious effect of oxygen is that at high concentrations, this dastardly gas increases the flammability and combustibility of any matter it is in contact with. This is why people on supplemental oxygen are encouraged not to smoke and avoid open flames. Unfortunately, this discovery was not found by controlled experimentation, but by hard knocks.The discovery of high oxygen’s combustion inducing properties were first documented by Soviet scientists during the Cold War’s famed “space race” between the United States and USSR. On the 23rd of March, 1961 Soviet Cosmonaut Valentin Bondarenko was undergoing day ten of a 15 day endurance experiment in a low pressure altitude chamber that was filled with at least 50% O2. Bondarenko was cleaning himself with a cotton ball, which he casually threw away. The cotton ball landed on a hot plate the cosmonaut was using to make tea, which quickly caught fire. Bondarenko attempted to put out the flame with the sleeve of his coveralls, which in the rich oxygen environment also caught fire. Within a minute Bondarenko and the entire chamber was awash in flames. The chamber door could not be opened due to the pressure difference cause by the fire, and doctors had to watch in horror as the flames continued until the oxygen in the chamber was exhausted a half hour later. Amazingly Bondarenko was still alive despite suffering 3rd degree burns over 100% of his body. He died 16 hours later, and was awarded the Order of the Red Star for his sacrifice to science.The accident was quickly covered up by the Soviet government, with files destroyed, official rosters erased, and even photographs airbrushed to erase Bondarenko from official history. While news of the accident never reached the Americans, several papers had been written on the dangers of high oxygen levels. Regardless, NASA did little to prepare for such an accident, deciding to tempt Murphy’s Law instead. On January 27th, 1967 the crew of Apollo I were conducting routine tests on the ground when a similar accident occurred. Inside the command capsule of the spacecraft, which was pressurized with 100% O2, “Gus” Grissom, Ed White, and Roger Chaffee were conducting a simulated launch when an errant electrical arc sparked a massive fire which engulfed the capsule in less than 17 seconds. Similar to the Bondarenko accident, the fire caused a change in the pressure gradient between the cabin and outside air, which sealed the capsule’s hatch shut. When the cabin finally depressurized, they found Ed White’s charred corpse still beside the hatch, where he had died trying to force it open. The other bodies were literally fuzed to cabin interior as the nylon of their spacesuits and gear had melted them in place. Investigation into the accident determined that the fire was sparked by faulty electronic wiring. Due to the 100% O2 interior, the fire spread quickly using the cabin’s 70 lbs of non-metallic flammable material as fuel, as well as the clothing and equipment of the astronauts. After the Apollo I accident the Apollo spacecraft was overhauled and redesigned, using less flammable materials to reduce the risk of fire, and a major overhaul of the ships wiring and electrical systems. Cabin oxygen content was also lowered to 60% O2 at launch. -- source link
Tumblr Blog : peashooter85.tumblr.com
#history#science#oxygen#death#space race#nasa#astronauts#cosmonauts#accidents#fire#chemistry#apollo i#space exploration