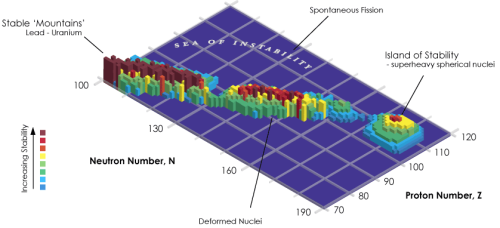
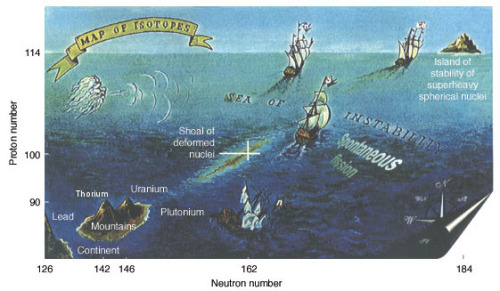
Island of StabilityIn nuclear physics, the island of stability is defined as “the predict
Island of StabilityIn nuclear physics, the island of stability is defined as “the prediction that a set of heavy isotopes with a near magic number of protons and neutrons will temporarily reverse the trend of decreasing stability in elements heavier than uranium”. Elements beyond rutherfordium (element 104) are considered superheavy elements, and the most long-lived ones are expected to exist on the so called island of stability with extremely long half-lives.While adding more nucleons tends to make elements unstable, the stability of elements doesn’t depend only on the number of protons and neutrons in its nucleus, but also the arrangement of said protons and neutrons. This is where the magic numbers come in: for protons 2, 8, 20, 28, 50, 82, 114, 120, 126 and for neutrons 2, 8, 20, 28, 50, 82, 126, 184. Evidence of these magic numbers can be seen in naturally occurring stable elements. In fact, lead, the last stable element, is considered to be double magic, with 82 protons and 126 neutrons (specifically lead-208). However, despite all this, the existence of superheavy and relatively stable isotopes has not yet been demonstrated. Some physicists think that the half-lives of elements on the island of stability will only be on the order of minutes, hours, or days, while others theorize they will be on the order of 109 years. Stability is relative - none of the elements past lead are ever expected to be completely stable.Sources: (Top Image) ( 2 ) (Bottom Image) ( 4 ) -- source link
Tumblr Blog : materialsscienceandengineering.tumblr.com
#materials science#science#elements#mymsepost