New generation of cancer-preventing vaccines could wipe out tumors before they formBy Jocelyn K
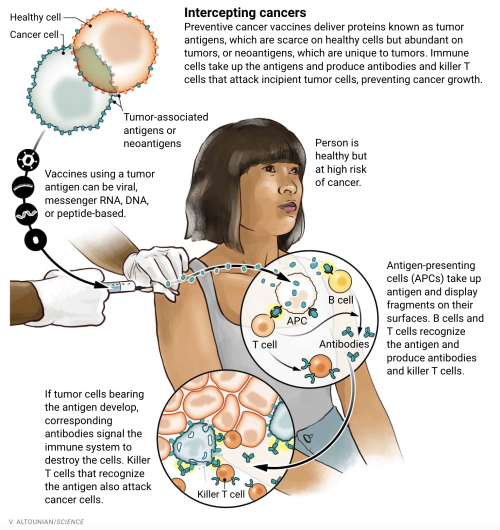
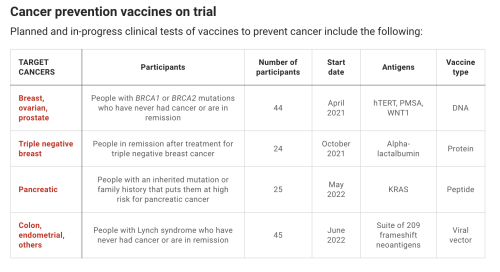
New generation of cancer-preventing vaccines could wipe out tumors before they formBy Jocelyn Kaiser (Science). doi: 10.1126/science.abq3411.When Dave Dubin learned at age 29 that he had colon cancer, it wasnt a big surprise. His grandfather and father had both survived the disease. “It was almost the Dubin way, and we just went on,” Dubin says. He had surgery and chemotherapy, but his cancer came back 10 years later. Genetic testing finally found an explanation for his family’s trials: a mutation in a DNA repair gene that lets genetic errors pile up in dividing cells. The disease, Lynch syndrome, comes with up to a 70% lifetime risk of cancer.Dubin, 55, gets annual colonoscopies, endoscopies, and imaging scans, which caught a third cancer, in his kidney. His eldest son, Zach Dubin, 26, inherited the DNA repair mutation and also regularly gets checked for cancer. “It’s no fun. Nobody enjoys it,” Dave Dubin says—not the 2-day colonoscopy prep and procedure, nor the worrying about possible tumors. The disease also turned him into an activist. He and his family in Haworth, New Jersey, launched a nonprofit, AliveAndKickn, to promote research and awareness of Lynch syndrome, which affects an estimated 1.1 million people in the United States.“There is a lot of anxiety in this patient population,” says oncologist and geneticist Eduardo Vilar-Sanchez of the MD Anderson Cancer Center. “It is a big psychological burden.” In hopes of easing that strain, Vilar-Sanchez will soon lead a clinical trial of a vaccine to prevent or at least delay Lynch-related cancers. If it works, Dave Dubin says, “it could be huge.”Vaccines to prevent certain types of cancer already exist. They target viruses: hepatitis B virus, which can trigger liver cancer, and human papillomavirus, which causes cervical and some other cancers. But most cancers are not caused by viruses. The Lynch vaccine trial will be one of the first clinical tests of a vaccine to prevent nonviral cancers.The idea is to deliver into the body bits of proteins, or antigens, from cancer cells to stimulate the immune system to attack any incipient tumors. The concept isn’t new, and it has faced skepticism. A decade ago, a Nature editorial dismissed a prominent breast cancer advocacy group’s goal of developing a preventive vaccine by 2020 as “misguided,” in part because of the genetic complexity of tumors. The editorial called the goal an “objective that science cannot yet deliver.” But now, a few teams—including one funded by the same advocacy group, the National Breast Cancer Coalition (NBCC)—are poised to test preventive vaccines, in some cases in healthy people at high genetic risk for breast and other cancers. Their efforts have been propelled by new insights into the genetic changes in early cancers, along with the recognition that because even nascent tumors can suppress the immune system, the vaccines should work best in healthy people who have never had cancer.Researchers are trying out several vaccine strategies. Some use so-called tumor antigens, molecular markers that are scarce on healthy cells but plentiful on cancer cells. The Lynch vaccine instead targets “neoantigens,” a potent type of antigen only found on tumor cells. Some deploy just a single antigen whereas others use a large number, in a bid to broadly shield against cancer. The best approach is unclear, and developers also face the difficult challenge of measuring success without waiting decades for healthy people to develop cancers.Early trials are yielding glimmers of promise. If the idea works to prevent one or a few cancers, it could be extended to meet an ambitious goal suggested by President Joe Biden: developing a vaccine that could prevent many types of cancer, modeled on the messenger RNA (mRNA) vaccines that have helped fight the COVID-19 pandemic. “We are a long way from a general vaccine” to prevent cancer, says medical oncologist Shizuko Sei of the National Cancer Institute’s Division of Cancer Prevention. “But it could be in the distant future. It’s a stepwise approach.”EFFORTS TO HARNESS the immune system to fight cancer have a long history. In the 1890s, physician William Coley reported that injections of bacterial toxins—a vaccine of sorts—sometimes shrank patients’ tumors, apparently by stimulating the immune system. Decades later, researchers discovered that immune cells called T cells could recognize tumor antigens as foreign and attack cancers. This finding led to two classes of approved therapies: drugs that lift molecular brakes on T cells so they can intensify their anticancer attack, and T cells engineered to home in on cancer cells. Both kinds of treatment have had striking success against certain cancers.A third type of immunotherapy, vaccines to treat cancer, has lagged. Efforts took off in the early 1990s, when researchers began to tally dozens of tumor antigens that might rouse a patient’s immune defenses. Often these antigens are proteins that cancer cells use to grow or spread, so the antigens are good markers of cancer cells.But despite promising data from animal experiments, most treatment vaccines failed to halt tumor growth in people. Because tumor-associated antigens can also be present in scant quantities on normal cells, the immune system tends to ignore them. The chemotherapy or other harsh treatments cancer patients receive also weaken their immune response, and tumors are protected by their “microenvironment”—surrounding cells and molecules that suppress killer T cells and block them from entering tumors. The only approved treatment vaccine, for advanced prostate cancer, extends life by just 4 months.Some scientists thought cancer vaccines might work better to prevent rather than treat the disease. One proponent was University of Pittsburgh cancer immunologist Olivera Finn, whose team in 1989 discovered the first tumor-associated antigen: a version of MUC1, a sugar-laden cell-surface protein. The altered version dots many types of cancer cells.Finn developed a vaccine consisting of short stretches of MUC1. In the first study of a preventive vaccine in healthy people, she tested safety in 39 people who had previously had precancerous colon polyps, which put them at elevated risk for colon cancer. In 2013, her team reported 17 had a strong immune response, with much higher levels of antibodies to the tumor version of MUC1 than previously seen in cancer patients who got the vaccine as treatment. The other 22 people, who didn’t make antibodies, had immune-suppressing cells in their blood, apparently lingering from their removed polyps, Finn says.The trial’s modest success led to a larger, placebo-controlled trial to see whether the vaccine prevented new polyps in people who had had them removed. This time, just 11 of 53 participants who received the vaccine produced plentiful antibodies, possibly because the patients’ immune-suppressing polyps had been removed only recently. But among the 11 responders, only three had polyps recur within 1 year of receiving the vaccine, compared with 31 of 47 participants in a placebo group, Finn’s team reports in a paper submitted to a journal.“It was very encouraging,” Finn says. “When you have no recurrence in responders, you know the vaccine is working.” Adding a treatment that blocks immune-suppressing cells may boost response rates, she says. Her team now plans MUC1 vaccine trials for several precancerous conditions.ONE DRAWBACK of Finn’s vaccine strategy is that the short proteins, or peptides, it contains mainly trigger one arm of the immune system: the B cells that make antibodies. “For immunity against cancer we really need to mobilize T cells,” says cancer immunologist Robert Vonderheide, director of Penn Medicine’s Abramson Cancer Center. That’s best done by injecting the genetic instructions for the antigen rather than the antigen itself. Special immune cells then take up the DNA or RNA, manufacture the antigen, chop it up, and display bits tailored to that person’s immune system on their cell surfaces. These antigen-presenting cells then teach T cells to recognize and kill tumor cells.Vonderheide’s team is testing a DNA-based vaccine targeting a different antigen that marks many tumors: hTERT, a small chunk of telomerase, an enzyme that protects chromosomes as cancer cells proliferate.Results of a trial testing the vaccine’s safety in 93 patients in remission after treatment for various cancers were encouraging. All but four people made T cells that home in on hTERT, the team reported in the Journal for ImmunoTherapy of Cancer in July 2021. And there was a hint the vaccine was warding off cancer. Among the 34 people who had had pancreatic cancer, 41% were still cancer free after 18 months. In other pancreatic cancer patients in remission, their tumor reappears within an average of 12 months.The Penn team is now studying safety and immune responses to the vaccine in 16 people in remission from previous cancers who have inherited mutations in BRCA1 or BRCA2, relatively common cancer genes that raise risk for breast and some other cancers. Next year, the researchers expect to give the vaccine to 28 people with BRCA mutations who have never had cancer.But because hTERT is found on some normal cells as well as cancerous ones, a vaccine could trigger an autoimmune attack on healthy cells, suggests immunologist Vincent Tuohy of the Cleveland Clinic. He has devised a breast cancer prevention vaccine that may be safer because it contains a breast cell protein called alpha-lactalbumin that people only make during late pregnancy and breastfeeding. Production of the protein also occurs in triple negative breast cancer, an aggressive form of the disease. Tuohy’s team is testing whether his protein vaccine can stimulate an immune response in 24 women who have been treated for triple negative breast cancer and have no plans to get pregnant. The next step, he says, will be a trial in healthy women with BRCA1 mutations, who are prone to this cancer type.Other teams hope to offer broader protection against breast cancer. Undeterred by being called “misguided” in 2012, NBCC is close to testing a breast cancer vaccine, initially in healthy breast cancer survivors. The advocacy group’s president, Fran Visco, says it set the ambitious goal because it was “frustrated with the lack of innovation in breast cancer.” With scientist partners, it has settled on a vaccine that combines six tumor antigens, including hTERT and MUC1. “We don’t know what type of breast cancer a woman is going to get,” explains trial leader Keith Knutson, an immunologist at the Mayo Clinic. Multipronged vaccines “are probably going to be more effective than vaccines targeting one individual protein,” says cancer immunologist Nora Disis of the University of Washington, Seattle, who is developing such a vaccine to prevent colon cancer.AS SOME TEAMS are trying to broaden the immune response triggered by cancer vaccines, others want to make it safer and more precise by targeting neoantigens, only found on cancer cells. Those efforts have accelerated over the past decade thanks to a surge in tumor genome sequencing, which has revealed a flood of neoantigens. Some drive cancer growth, whereas others have no apparent function. Most are unique to an individual cancer—an obstacle for developing preventive vaccines, which have to target markers that can be predicted in advance.Some neoantigens reliably appear on many people’s tumors, however. For instance, pancreatic cancer is almost always triggered by mutations in a growth protein called KRAS, which give rise to a predictable set of neoantigens. This spring, Johns Hopkins University immunologist Elizabeth Jaffee and colleague Neeha Zaidi will begin to safety test a vaccine containing mutated KRAS peptides in 25 men and women who haven’t had cancer but are at high risk because of an inherited mutation or family history. KRAS is like pancreatic cancer’s Achilles’ heel, Jaffee says: It’s the first of several genes to get mutated. As a result, the team hopes early tumor cells won’t be able to evade the vaccine by ditching KRAS and finding another way to grow.Lynch syndrome cancers also sport a predictable set of neoantigens. That’s because patients’ DNA repair problem leads to “frameshift” mutations, which shift how a cell’s proteinmaking machinery reads a gene, scrambling the resulting protein in a consistent way. A peptide vaccine containing a few of these neoantigens, which was developed by a German team, caused no serious side effects when tested in people with cancer. A similar vaccine designed for mice with Lynch syndrome reduced tumor growth, researchers reported in July 2021 in Gastroenterology.The vaccine Vilar-Sanchez’s team will test is more ambitious: It consists of viruses modified to carry DNA for a whopping 209 frameshift neoantigens found in Lynch tumors. People’s immune systems vary in how they respond to specific neoantigens, and different individuals’ tumors won’t all make the same set. “Therefore, the best [approach] is to have many,” says Elisa Scarselli, chief scientific officer of Nouscom, an Italian company developing the vaccine.The vaccine is also being developed as treatment, and in an early test Nouscom is giving it along with an immunotherapy drug to patients who have metastatic cancers with frameshift mutations like those in Lynch syndrome. At a meeting in fall 2021, the company reported the treatment shrank tumors in seven of the first 12 patients. “We really believe we will see even more immunogenicity in healthy carriers of Lynch disease” because they should have stronger immune systems, Scarselli says.Vilar-Sanchez’s trial, beginning within a few months, will give the vaccine to 45 volunteers with Lynch syndrome—both people in remission after cancer treatment and others who have never had tumors. Investigators will assess whether the vaccine stimulates an immune response and has any apparent effect on polyps or tumor formation.If the results look good, the next step will be a randomized study of hundreds of patients over perhaps 5 to 10 years. “There’s a lot to be gained” if the vaccine works, Vilar-Sanchez says. “A cancer vaccine is not going to reduce the risk to zero, but it could impact how often we perform screening.” It could also help patients decide whether to have a hysterectomy to prevent endometrial cancers, which are common in people with Lynch syndrome.All prevention vaccines would face a long road to regulatory approval if researchers must wait for tumors to appear to judge the vaccine’s efficacy. So they will also look for surrogate measures of protection, such as reduced growth of polyps in people prone to colon cancer. For breast cancer, researchers don’t have biomarkers yet but hope to find them, perhaps a change in blood-borne immune cells or breast tissue, Vonderheide says.“We have to be smart enough to present to the FDA [U.S. Food and Drug Administration] a biomarker of success,” Vonderheide says. “This is formidable. But we’re inspired because the impact will be massive.”WHATEVER THEIR PREFERRED antigens, many scientists expect to model their next preventive vaccines on the leading COVID-19 vaccines, which use a lipid particle to ferry mRNA for antigens into cells. mRNA vaccines are easier to make and deliver than DNA or viral vaccines, and the pandemic has shown they’re generally safe and stimulate a strong response. “The fact that mRNA vaccines have shown safety in billions of healthy people of all ages makes [mRNA] a very good platform” for preventive cancer vaccines, Jaffee says.The White House is gunning for mRNA vaccines to prevent cancer, too. They are on the list of potential projects for a reignited Cancer Moonshot and the new high-risk, high-reward research agency, the Advanced Research Projects Agency for Health (ARPA-H). A concept paper for ARPA-H puts the goal this way: “Use mRNA vaccines to teach the immune system to recognize 50 common genetic mutations that drive cancers, so that the body will wipe out cancer cells when they first arise.”That description raises some eyebrows. “That would be heroic,” Finn says, because the vaccine antigens would have to cover not only a huge number of cancer mutations, but also “the incredible genetic diversity” in individuals’ immune responses. “Not impossible but not simple,” she says.Clinical geneticist Steven Lipkin of Weill Cornell Medicine, who works on Lynch syndrome vaccines, is cautiously optimistic, noting that a vaccine that cut the rates of the most common cancers “by say one-third or one-half in a large number of people would be a tremendous benefit.”One team is already testing a multicancer prevention vaccine—not yet in people, but in dogs. In a 5-year trial, a team is giving 400 middle-age dogs a vaccine that contains 31 antigens from eight common dog cancers. (Another 400 dogs are getting a placebo vaccine.) It relies on RNA neoantigens, little-studied molecules that result from RNA processing errors rather than mutations in DNA. They are far more abundant than DNA neoantigens in dogs and people, and are “highly immunogenic,” says developer and biochemist Stephen Johnston of the Biodesign Institute at Arizona State University, Tempe. If they prove effective, they might make it easier to reach the White House’s goal of developing a pancancer human vaccine, he says.Another proponent of a universal cancer prevention vaccine is Johns Hopkins cancer geneticist Bert Vogelstein. He notes that sequencing has shown “a relatively small number of genes are involved in most cancers,” suggesting a limited number of antigens could lead to broad protection. Such a vaccine “seems like science fiction,” Vogelstein says, but “a concerted effort by many labs” might succeed. Sei agrees: “That’s not crazy. That’s possible.”For Dave Dubin, even a narrower success—a Lynch syndrome vaccine—“could be game-changing,” he says, if it meant fewer cancer screenings and no more major surgeries. “The goal would be almost to live a normal life.” -- source link
Tumblr Blog : mednerds.tumblr.com
#science#medicine#academia#health#medblr#oncology